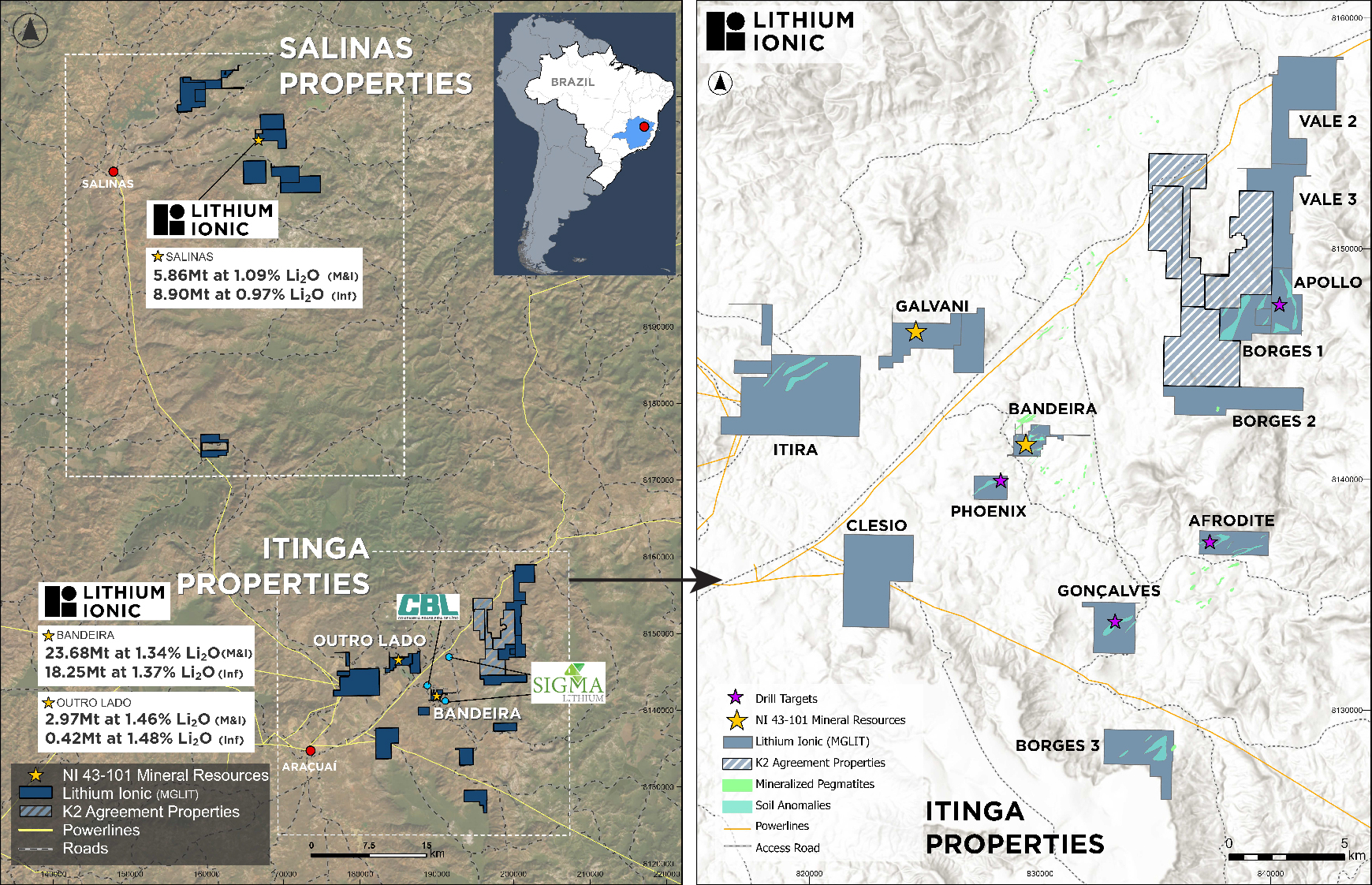
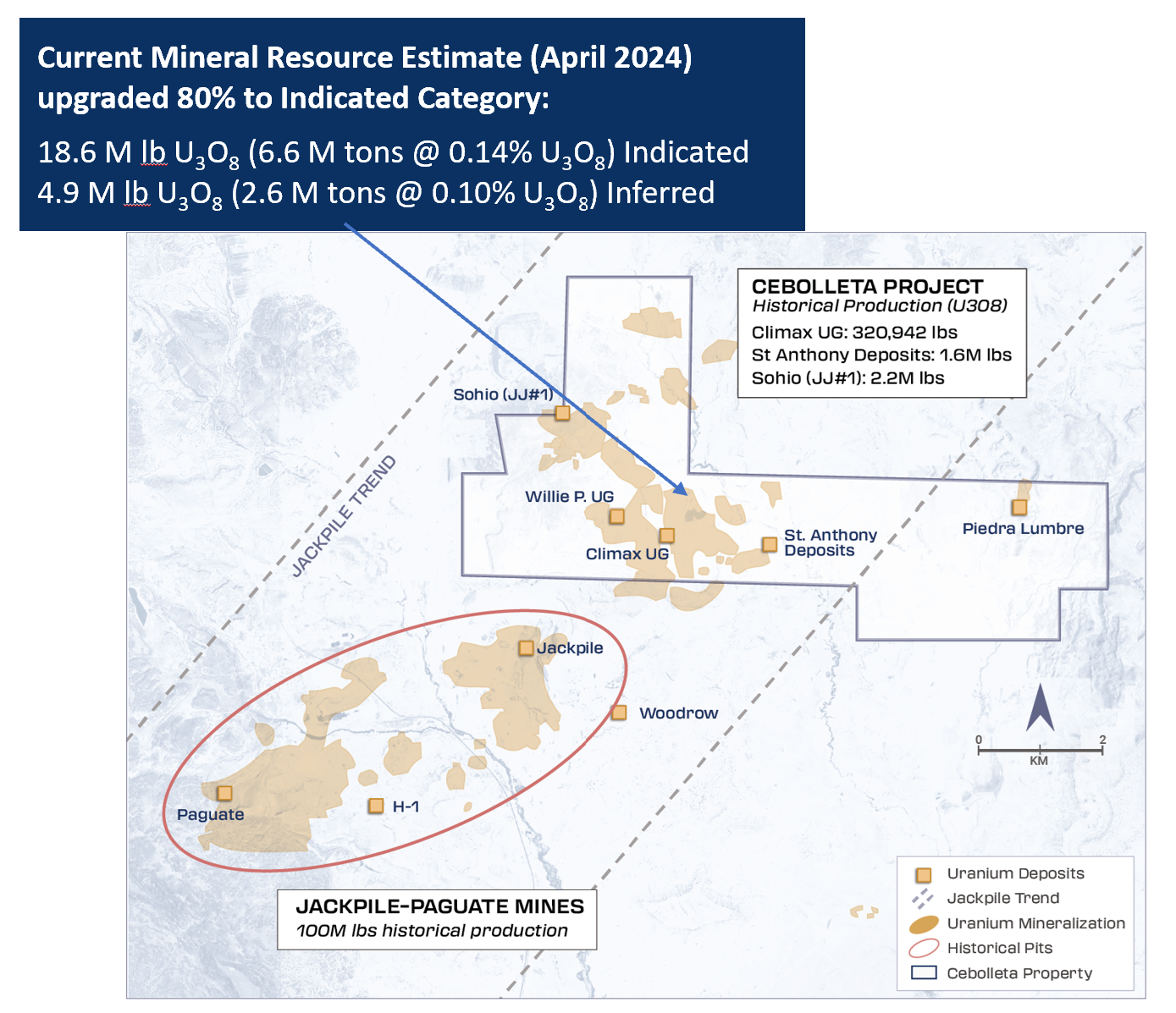
EGFR Non-Small Cell Lung Cancer Clinical Trial Pipeline Appears Robust With 37+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The increase in the prevalence of Non-Small Cell Lung Cancer (NSCLC) drives market growth by boosting demand for innovative treatments, and personalized medicine approaches. According to a study, a total of 456 studies were included, reporting 30,466 patients with EGFR mutations among 115,815 NSCLC patients. The overall pooled prevalence for EGFR mutations was 32.3%, ranging from 38.4% in China to 14.1% in Europe. This surge also stimulates investment in research, technological advancements, and healthcare infrastructure, expanding market opportunities globally.
New York, USA, July 03, 2024 (GLOBE NEWSWIRE) — EGFR Non-Small Cell Lung Cancer Clinical Trial Pipeline Appears Robust With 37+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The increase in the prevalence of Non-Small Cell Lung Cancer (NSCLC) drives market growth by boosting demand for innovative treatments, and personalized medicine approaches. According to a study, a total of 456 studies were included, reporting 30,466 patients with EGFR mutations among 115,815 NSCLC patients. The overall pooled prevalence for EGFR mutations was 32.3%, ranging from 38.4% in China to 14.1% in Europe. This surge also stimulates investment in research, technological advancements, and healthcare infrastructure, expanding market opportunities globally.
DelveInsight’s ‘EGFR Non-Small Cell Lung Cancer Pipeline Insight 2024‘ report provides comprehensive global coverage of pipeline EGFR non-small cell lung cancer therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the EGFR non-small cell lung cancer pipeline domain.
Key Takeaways from the EGFR Non-Small Cell Lung Cancer Pipeline Report
- DelveInsight’s EGFR non-small cell lung cancer pipeline report depicts a robust space with 37+ active players working to develop 40+ pipeline therapies for EGFR non-small cell lung cancer treatment.
- Key EGFR non-small cell lung cancer companies such as Cullinan Oncology, Suzhou Puhe Pharmaceutical Technology, Taiho Pharmaceutical, ORIC Pharmaceuticals, Nuvalent, Shanghai Junshi Biosciences, Janux Therapeutics, HK inno.N, Betta Pharmaceuticals, Bridge Biotherapeutics, Sorrento Therapeutics, Bayer, Avistone Pharmaceuticals, and others are evaluating new EGFR non-small cell lung cancer drugs to improve the treatment landscape.
- Promising EGFR non-small cell lung cancer pipeline therapies such as Zipalertinib, YK-029A, TAS3351, ORIC-114, NVL-330, JS-113, JANX-008, IN-119873, MCLA-129, BBT-176, Abivertinib, BAY2927088, FPI-2068, PLB1004, and others are under different phases of EGFR non-small cell lung cancer clinical trials.
- In May 2024, Affimed N.V. announced that an update from the Company’s AFM 24-102 study in advanced EGFR wild-type (EGFRwt) non-small cell lung cancer will be presented at the annual meeting of the American Society of Clinical Oncology (ASCO) to be held in Chicago on May 31 – June 4, 2024.
- In April 2024, ORIC Pharmaceuticals, Inc. announced the completion of the dose escalation portion of the Phase Ib trial of ORIC-114 in patients with advanced solid tumors with EGFR and HER2 exon 20 alterations or HER2 amplifications. Based upon these data, ORIC selected the two provisional recommended Phase II dose (RP2D) levels of ORIC-114 at 80 mg and 120 mg QD, which are being further evaluated in three dose expansion cohorts for dose optimization and final RP2D selection. These expansion cohorts have now been initiated in patients with non-small cell lung cancer (NSCLC) with EGFR exon 20 (EGFR exon 20 inhibitor naïve), HER2 exon 20, or EGFR atypical mutations. The company also announced the initiation of an extension cohort for the treatment of patients with first-line, treatment-naïve EGFR exon 20 NSCLC.
- In April 2024, Nuvalent, Inc. announced the presentation of new preclinical data for its novel HER2-selective inhibitor, NVL-330. The poster was presented at the American Association for Cancer Research (AACR) Annual Meeting that took place from April 5 – 10 in San Diego.
- In February 2024, Bayer announced that the US Food and Drug Administration (FDA) had granted Breakthrough Therapy designation for BAY 2927088 for the treatment of adult patients with unresectable or metastatic non-small cell lung cancer whose tumors have activating HER2 (ERBB2) mutations, and who have received a prior systemic therapy.
- In December 2023, Merus N.V., a clinical-stage oncology company developing innovative, full-length multispecific antibodies, announced updated interim clinical data on MCLA-129 from ongoing expansion cohorts in non-small cell lung cancer and in previously treated head and neck squamous cell carcinoma (HNSCC) were presented at the European Society for Medical Oncology (ESMO) Asia Congress 2023.
- In December 2023, Daiichi Sankyo and Merck, known as MSD outside of the United States and Canada, announced that the US Food and Drug Administration (FDA) has accepted and granted Priority Review to the Biologics License Application (BLA) for patritumab deruxtecan (HER3-DXd) for the treatment of adult patients with locally advanced or metastatic EGFR-mutated non-small cell lung cancer previously treated with two or more systemic therapies.
Request a sample and discover the recent advances in EGFR non-small cell lung cancer treatment drugs @ EGFR Non-Small Cell Lung Cancer Pipeline Report
The EGFR non-small cell lung cancer pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage EGFR non-small cell lung cancer drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the EGFR non-small cell lung cancer clinical trial landscape.
EGFR Non-Small Cell Lung Cancer Overview
Lung cancer stands as a prominent contributor to cancer-related fatalities in both genders, with treatment choices for advanced stages being notably scarce. Non-small cell lung cancer (NSCLC), encompassing roughly 75% of cases, presents a formidable treatment challenge due to insufficient comprehension of its underlying pathological processes. Genetic and regulatory irregularities governing cell survival pathways have been uncovered, facilitating tumor growth by impeding cell demise, fostering cellular proliferation, and triggering tumor formation. Among these revelations is the identification of epidermal growth factor receptor (EGFR) as a significant player.
EGFR is part of the erbB family, a group of closely related receptor tyrosine kinases comprising erbB1 (also called EGFR), erbB2 (HER2), erbB3, and erbB4. While they share similar structures, each member possesses distinct characteristics, particularly in tyrosine kinase activity. EGFR consists of an extracellular ligand binding domain, a transmembrane segment, and intracellular tyrosine kinase and regulatory domains. Upon binding to specific ligands like epidermal growth factor (EGF), EGFR undergoes a conformational shift, leading to phosphorylation of its intracellular domain. This event triggers downstream signal transduction through various pathways such as Raf1-extracellular signal-regulated kinase, PI3K/Akt, and signal transducer and activator of transcription (STAT) factors. The outcome, whether cell proliferation or inhibition of apoptosis, depends on the activated pathway.
Three generations of EGFR-TKIs have been approved for treating NSCLC patients carrying EGFR mutations across various clinical scenarios. The initial generation (including gefitinib, erlotinib, and icotinib) and the subsequent generation (comprising afatinib and dacomitinib) EGFR-TKIs have displayed considerable clinical advantages for advanced NSCLC patients harboring Ex19del and L858R mutations. Despite the promising initial responses to these first- and second-generation EGFR-TKIs, a significant proportion of patients tend to develop acquired resistance within a span of 9 to 14 months, either during or post-EGFR-TKI therapy.
Find out more about EGFR non-small cell lung cancer treatment drugs @ Drugs for EGFR Non-Small Cell Lung Cancer Treatment
A snapshot of the EGFR Non-Small Cell Lung Cancer Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | RoA |
| Zipalertinib | Cullinan Oncology | Phase III | Oral |
| YK-029A | Suzhou Puhe Pharmaceutical Technology | Phase III | Oral |
| ORIC-114 | ORIC Pharmaceuticals | Phase I/II | Oral |
| TAS3351 | Taiho Pharmaceutical | Phase I/II | Oral |
| MCLA-129 | Betta Pharmaceuticals | Phase I/II | Intravenous |
| BAY2927088 | Bayer | Phase I/II | Oral |
| NVL-330 | Nuvalent | Preclinical | Oral |
Learn more about the emerging EGFR non-small cell lung cancer pipeline therapies @ EGFR Non-Small Cell Lung Cancer Clinical Trials
EGFR Non-Small Cell Lung Cancer Therapeutics Assessment
The EGFR non-small cell lung cancer pipeline report proffers an integral view of the EGFR non-small cell lung cancer emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the EGFR Non-Small Cell Lung Cancer Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Key EGFR Non-Small Cell Lung Cancer Companies: Cullinan Oncology, Suzhou Puhe Pharmaceutical Technology, Taiho Pharmaceutical, ORIC Pharmaceuticals, Nuvalent, Shanghai Junshi Biosciences, Janux Therapeutics, HK inno.N, Betta Pharmaceuticals, Bridge Biotherapeutics, Sorrento Therapeutics, Bayer, Avistone Pharmaceuticals, and others
- Key EGFR Non-Small Cell Lung Cancer Pipeline Therapies: Zipalertinib, YK-029A, TAS3351, ORIC-114, NVL-330, JS-113, JANX-008, IN-119873, MCLA-129, BBT-176, Abivertinib, BAY2927088, FPI-2068, PLB1004, and others
Dive deep into rich insights for new drugs for EGFR non-small cell lung cancer treatment, visit @ EGFR Non-Small Cell Lung Cancer Drugs
Table of Contents
| 1. | EGFR Non-Small Cell Lung Cancer Pipeline Report Introduction |
| 2. | EGFR Non-Small Cell Lung Cancer Pipeline Report Executive Summary |
| 3. | EGFR Non-Small Cell Lung Cancer Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | EGFR Non-Small Cell Lung Cancer Clinical Trial Therapeutics |
| 6. | EGFR Non-Small Cell Lung Cancer Pipeline: Late-Stage Products (Pre-registration) |
| 7. | EGFR Non-Small Cell Lung Cancer Pipeline: Late-Stage Products (Phase III) |
| 8. | EGFR Non-Small Cell Lung Cancer Pipeline: Mid-Stage Products (Phase II) |
| 9. | EGFR Non-Small Cell Lung Cancer Pipeline: Early-Stage Products (Phase I) |
| 10. | EGFR Non-Small Cell Lung Cancer Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the EGFR Non-Small Cell Lung Cancer Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the EGFR Non-Small Cell Lung Cancer Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the EGFR non-small cell lung cancer pipeline therapeutics, reach out @ EGFR Non-Small Cell Lung Cancer Treatment Drugs
Related Reports
Non-small Cell Lung Cancer Epidemiology Forecast
Non-small Cell Lung Cancer Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted non-small cell lung cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Non-small Cell Lung Cancer Pipeline
Non-small Cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key non-small cell lung cancer companies, including BridgeBio Pharma, Daiichi Sankyo, EMD Serono, Merck, BridgeBio Pharma, Abbvie, Pfizer, Eli Lilly and Company BioNTech SE, Shenzhen TargetRx, Taiho Pharmaceutical, Chong Kun Dang, Bristol Myers Squibb, Innovent Biologics, Xuanzhu Biopharmaceutical, Bayer, GeneScience Pharmaceuticals, InventisBio, Apollomics, Imugene, Ono Pharmaceutical, Pierre Fabre, Jiangsu Hengrui Medicine Co., Bristol-Myers Squibb, Surface Oncology, Inhibrx, Sinocelltech, Mirati Therapeutics, REVOLUTION Medicines, Yong Shun Technology Development, Iovance Biotherapeutics, Galecto Biotech, among others.
HER2-mutant Non-Small Cell Lung Cancer Pipeline
HER2-mutant Non-Small Cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HER2-mutant non-small cell lung cancer companies, including Dizal Pharmaceuticals, Puma Biotechnology, AstraZeneca, Jiangsu Hengrui Medicine, among others.
Small-cell Lung Cancer Pipeline
Small-cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key small-cell lung cancer companies, including Ascentage Pharma, Merck & Co, AstraZeneca, Advenchen Laboratories, GlaxoSmithKline, Advanced Accelerator Applications, Trillium Therapeutics, Vernalis, Oncoceutics, NewBio Therapeutics, Wigen Biomedicine, Linton Pharm, Carrick Therapeutics, Xencor, Jiangsu HengRui Medicine, Aileron Therapeutics, Roche, Ipsen, Celgene, Lee’s Pharmaceutical Limited, AbbVie, G1 Therapeutics, Chipscreen Biosciences, Luye Pharma Group, Shanghai Henlius Biotech, CSPC ZhongQi Pharmaceutical Technology, Impact Therapeutics, among others.
Small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key advanced small cell lung cancer companies, including Ascentage Pharma, Merck & Co, AstraZeneca, Advenchen Laboratories, Advanced Accelerator Applications, Trillium Therapeutics, Wigen Biomedicine, Linton Pharm, Carrick Therapeutics, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com