Sarcopenia Market Anticipates Impressive Growth Trajectory Across 7MM During the Study Period (2020–2034) | DelveInsight
The sarcopenia market size shall grow during the forecast period (2024–2034) due to the launch of upcoming therapies and the increasing cases of sarcopenia. This sarcopenia treatment market is expected to increase at a significant rate.
New York, USA, Sept. 25, 2024 (GLOBE NEWSWIRE) — Sarcopenia Market Anticipates Impressive Growth Trajectory Across 7MM During the Study Period (2020–2034) | DelveInsight
The sarcopenia market size shall grow during the forecast period (2024–2034) due to the launch of upcoming therapies and the increasing cases of sarcopenia. This sarcopenia treatment market is expected to increase at a significant rate.
DelveInsight’s Sarcopenia Market Insights report includes a comprehensive understanding of current treatment practices, sarcopenia emerging drugs, market share of individual therapies, and current and forecasted sarcopenia market size from 2020 to 2034, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Sarcopenia Market Report
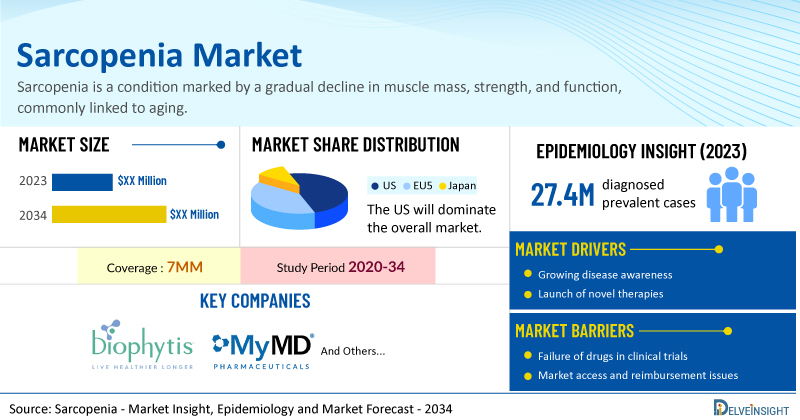
- According to DelveInsight’s analysis, sarcopenia market size in the 7MM is expected to grow at a significant CAGR by 2034.
- The total diagnosed prevalent cases of sarcopenia in the 7MM was 27.4 million cases in 2023 and this number is forecasted to climb at a significant CAGR in the research period (2020–2034).
- Prominent sarcopenia companies working in the domain, including Biophytis, MyMD Pharmaceuticals, Inc., and others, are actively working on innovative drugs for sarcopenia. These novel sarcopenia therapies are anticipated to enter the sarcopenia market in the forecast period and are expected to change the market.
- Some of the key therapies for sarcopenia treatment include Sarconeos (BIO101), MYMD-1, and others.
- In August 2024, TNF Pharmaceuticals, Inc. announced plans to move forward with its lead program, MYMD-1, into fully funded mid-stage clinical trials. The upcoming studies will further investigate the drug’s effectiveness in treating sarcopenia/frailty, following statistically significant positive outcomes from a previous Phase II trial.
Discover which therapies are expected to grab sarcopenia market share @ Sarcopenia Treatment Market Report
Sarcopenia Overview
Sarcopenia is a condition marked by a gradual decline in muscle mass, strength, and function, commonly linked to aging. It arises from a combination of physiological changes in the musculoskeletal system as people grow older. This condition is a major health issue due to its effects on physical performance, functional abilities, and overall health.
Sarcopenia stems from a mix of genetic factors, lifestyle habits, and age-related physiological changes, primarily involving reduced muscle mass and function. Hormonal changes, such as decreased levels of growth hormone, testosterone, and insulin-like growth factor 1, play a role in its progression. Factors like lack of physical activity, inadequate exercise, and poor nutrition accelerate muscle loss. Other contributing elements include chronic diseases, inflammation, neuromuscular issues, genetics, certain medications, hormonal imbalances, malabsorption, smoking, and excessive alcohol consumption.
Sarcopenia diagnosis requires various methods, including DEXA scans, anthropometry, MRI, and CT scans to assess muscle mass, as well as tests for grip strength, gait speed, and blood analyses for additional insights. However, establishing precise diagnostic criteria is complex due to differing measurement techniques and their intricacies.
Sarcopenia Epidemiology Segmentation
The sarcopenia epidemiology section provides insights into the historical and current sarcopenia patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The sarcopenia market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Sarcopenia Prevalence
- Gender-specific Diagnosed Sarcopenia Prevalence
- Age-specific Diagnosed Sarcopenia Prevalence
- Severity-specific Diagnosed Sarcopenia Prevalence
Download the report to understand which factors are driving sarcopenia epidemiology trends @ Sarcopenia Epidemiological Insights
Sarcopenia Treatment Market
As of now, there are no approved pharmacological treatments for sarcopenia. Consequently, the current recommendations for preventing and treating sarcopenia primarily focus on lifestyle changes. This underscores a notable gap in available treatments and highlights the urgent need for developing safe and effective therapies to address the issues faced by those with sarcopenia.
To manage hormonal imbalances and support overall musculoskeletal health, adequate protein intake, proper nutrition, vitamin D supplements, and sometimes hormone replacement therapy may be recommended by healthcare professionals. Although nutritional supplements can be beneficial, steroid hormones like dehydroepiandrosterone (DHEA), testosterone, and anabolic steroids have shown some positive effects, but their use is restricted due to potential adverse effects.
Although no specific drugs have been approved for sarcopenia, research is ongoing to explore potential pharmacological treatments. Medications such as selective androgen receptor modulators (SARMs) and myostatin inhibitors are being studied for their potential to enhance muscle growth and function.
Not everyone with sarcopenia will also experience sarcopenic obesity, although these conditions can occur together, particularly in older adults. The relationship between sarcopenia and obesity is complex, and the frequency of sarcopenic obesity varies across different populations. Some research suggests that obesity might increase the risk of developing sarcopenia.
Sarcopenic obesity, characterized by the combination of muscle loss and excess body fat, presents a distinctive challenge in treatment. The interaction between muscle depletion and increased fat can lead to greater risks of functional decline and metabolic problems. Managing this condition requires a comprehensive approach that focuses on both maintaining or restoring muscle mass and reducing excess body fat. Effective management typically involves customized exercise regimens and dietary strategies, aiming for a holistic intervention to enhance overall health.
Learn more about the FDA-approved drugs in sarcopenia therapeutics market @ Drugs for Sarcopenia Treatment
Emerging Sarcopenia Drugs and Companies
There are many innovative key sarcopenia companies involved in the development of promising products such as Biophytis (Sarconeos), MyMD Pharmaceuticals, Inc. (MYMD-1), and others.
Biophytis is developing Sarconeos (BIO101), an orally administered small molecule designed to treat neuromuscular diseases. By activating the MAS receptor in muscle cells, Sarconeos boosts biological resilience and helps maintain muscle function in conditions related to aging and muscle wasting. The MAS receptor plays a key role in the Renin-Angiotensin System, which regulates various physiological processes. Positive results from Phase II trials show that Sarconeos improves gait speed and is safe over 9 months. With global collaborations expected, the goal is to advance BIO101 to Phase III, potentially filling the gap in sarcopenia treatment and revolutionizing this therapeutic area. Currently, the drug is in Phase II of clinical development.
MYMD-1 is an innovative treatment aimed at tackling conditions associated with aging, like sarcopenia and depression, especially those related to age or inflammation. It also shows potential as a treatment for fibrosis and excessive cell growth. As a next-generation TNF-alpha inhibitor, which is MYMD-1 MoA, it is taken orally, providing a more convenient and safer option compared to current anti-TNF treatments. This advancement could offer significant benefits for patients who aren’t well served by existing TNF-alpha inhibitors. The drug is now in Phase II of clinical trials.
The anticipated launch of these emerging therapies are poised to transform the sarcopenia market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the sarcopenia market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about sarcopenia clinical trials, visit @ Sarcopenia Treatment Drugs
The sarcopenia market dynamics are anticipated to change in the coming years. Ongoing research into the causes, prevention, and treatment of sarcopenia, combined with the introduction of a new disease code (M62.84) in ICD-10-CM in October 2016, has led to a better understanding of the condition and heightened market recognition. The absence of FDA-approved sarcopenia treatments presents an exciting opportunity for focused research on developing precise therapies, while health authorities are moving closer to accepting physical performance-based and patient-reported outcome assessments for use in drug trials.
Furthermore, potential therapies are being investigated for the treatment of sarcopenia, and it is safe to predict that the treatment space will significantly impact the sarcopenia market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of the sarcopenia market in the 7MM.
However, several factors may impede the growth of the sarcopenia market. Even with a known etiology, the rapid loss of muscle mass and function typically associated with the “normal aging” process is compounded by the absence of standardized treatment guidelines, which poses a challenge. Strained healthcare resources may limit the ability to provide optimal care and interventions for individuals with sarcopenia, leading to increased healthcare costs due to its association with falls, fractures, and other health complications, thereby posing economic challenges to healthcare systems.
Moreover, sarcopenia treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, sarcopenia market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact sarcopenia treatment market growth.
| Sarcopenia Report Metrics | Details |
| Study Period | 2020–2034 |
| Sarcopenia Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Sarcopenia Companies | Biophytis, MyMD Pharmaceuticals, Inc., and others |
| Key Sarcopenia Therapies | Sarconeos (BIO101), MYMD-1, and others |
Scope of the Sarcopenia Market Report
- Sarcopenia Therapeutic Assessment: Sarcopenia current marketed and emerging therapies
- Sarcopenia Market Dynamics: Attribute Analysis of Emerging Sarcopenia Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Sarcopenia Market Access and Reimbursement
Discover more about sarcopenia drugs in different phases of development @ Sarcopenia Clinical Trials
Table of Contents
| 1. | Sarcopenia Market Key Insights |
| 2. | Sarcopenia Market Report Introduction |
| 3. | Sarcopenia Market Overview at a Glance |
| 4. | Sarcopenia Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Sarcopenia Treatment and Management |
| 7. | Sarcopenia Epidemiology and Patient Population |
| 8. | Sarcopenia Patient Journey |
| 9. | Marketed Sarcopenia Drugs |
| 10. | Emerging Sarcopenia Drugs |
| 11. | Seven Major Sarcopenia Market Analysis |
| 12. | Sarcopenia Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Sarcopenia Epidemiology Forecast
Sarcopenia Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted sarcopenia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Sarcopenia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key sarcopenia companies, including Oncocross, Rejuvenate Biomed, MyMD Pharmaceuticals, Biophytis, NMD Pharma, among others.
Myasthenia Gravis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key myasthenia gravis companies, including Viela Bio, UCB Pharma, Momenta Pharmaceuticals, Sanofi, Regeneron Pharmaceuticals, Ra Pharmaceuticals, Hoffmann-La Roche, Alexion Pharmaceuticals, Catalyst Pharmaceuticals, Harbour BioMed, Novartis, Takeda, DAS Therapeutics, RemeGen, Cartesian Therapeutics, Nanjing IASO Biotherapeutics, Cabaletta Bio, CytoDyn, Ahead Therapeutics, Toleranzia, Rallybio, among others.
Generalized Myasthenia Gravis Pipeline
Generalized Myasthenia Gravis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key generalized myasthenia gravis companies, including Biocon, Cartesian Therapeutics, UCB, Momenta Pharmaceuticals, HanAll Biopharma, Roche, Alexion, Novartis, Takeda, BioMarin, among others.
Generalized Myasthenia Gravis Market
Generalized Myasthenia Gravis Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key generalized myasthenia gravis companies, including UCB Biopharma, Argenx-Halozyme Therapeutics, Horizon Therapeutics, Hoffmann-La Roche, Janssen Research & Development, LLC, Immunovant Sciences GmbH, Sanofi, Cartesian Therapeutics, Takeda, DAS Therapeutics, Chugai Pharmaceutical, Inc., Alexion, Regeneron Pharmaceuticals, Ra Pharmaceuticals, Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com











