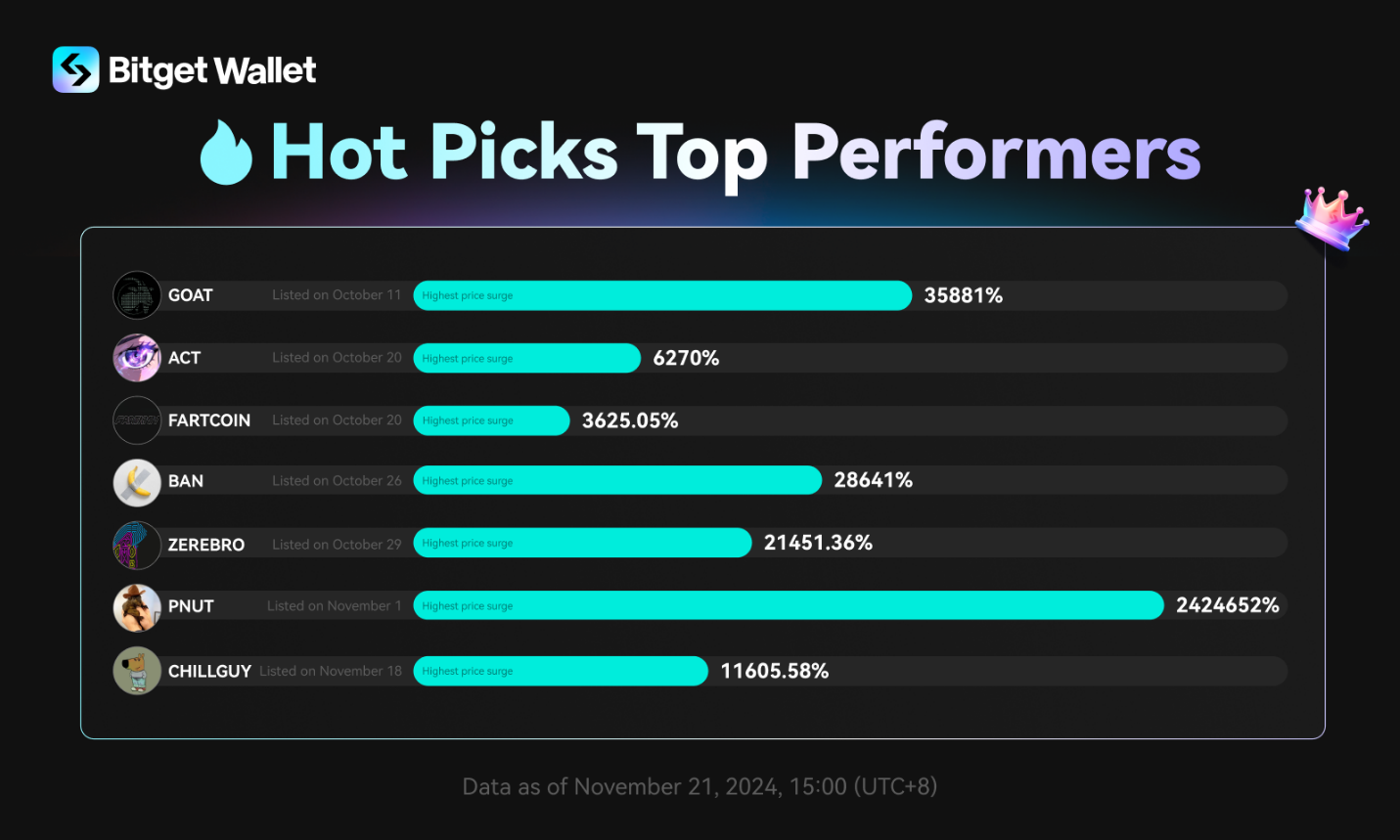
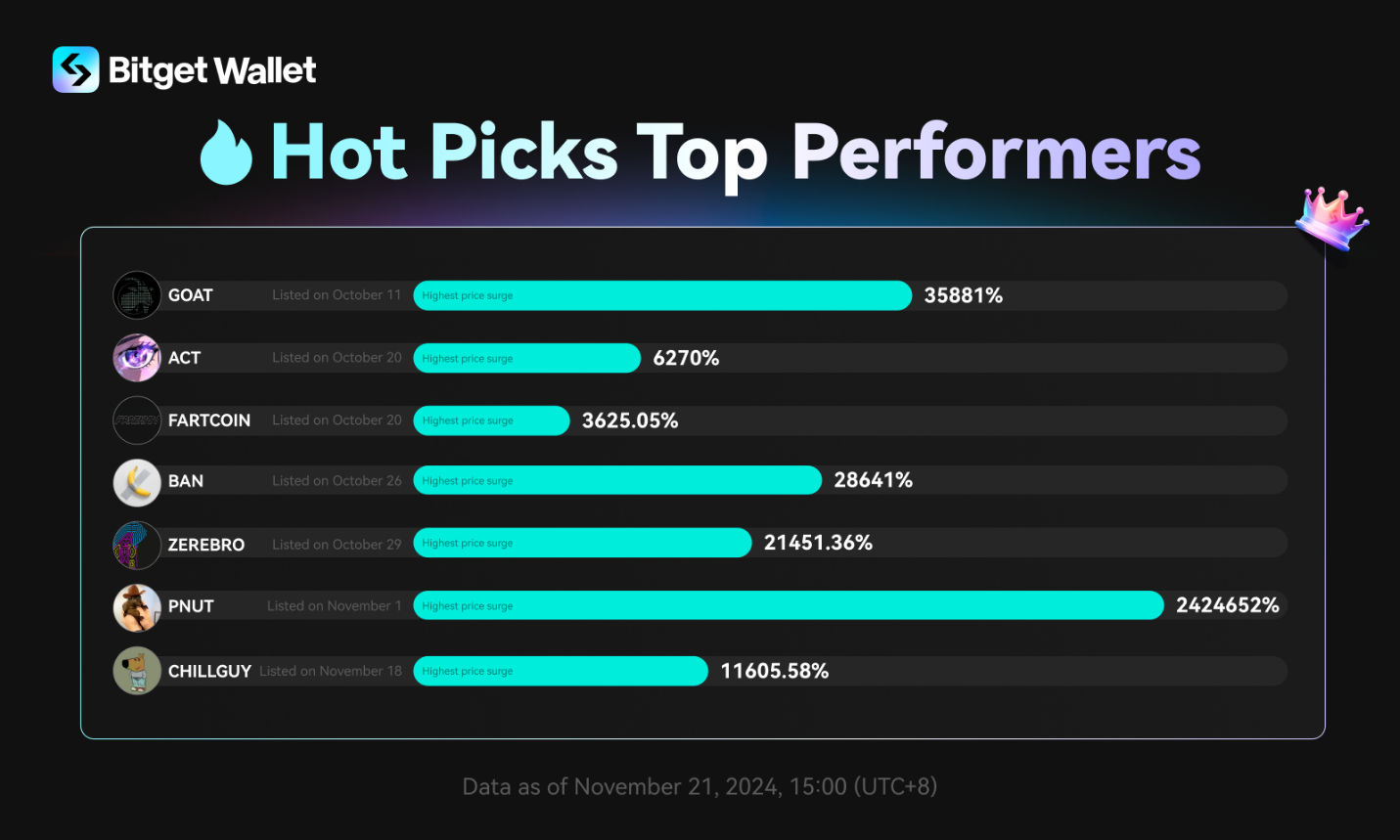
Valneva to Present and Hold Investor Meetings at Upcoming U.S. and European Healthcare Conferences
Saint-Herblain (France), November 12, 2024 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company, today announced that its senior management will present and participate in 1-on-1 meetings with institutional investors at upcoming investor conferences in the United States and Europe.
Chief Executive Officer Thomas Lingelbach and Chief Financial Officer Peter Bühler will host moderated “fireside chat” presentations to discuss the Company’s key value drivers and upcoming catalysts, which include multiple clinical data events in 2025, most notably key Phase 3 conclusions for VLA15, the Company’s Lyme disease vaccine candidate, which is partnered with Pfizer. If successful, Pfizer aims to submit applications for U.S. and European market authorization in 2026.
Guggenheim Inaugural Healthcare Innovation Conference
Date/Time: November 12, 3:30pm EST
Format: Fireside chat and investor meetings
Location: Boston, Massachusetts
Jefferies London Healthcare Conference
Date/Time: November 19, 8:00am GMT
Format: Fireside chat and investor meetings
Location: London, United Kingdom
Webcast Link: https://wsw.com/webcast/jeff315/valn/1852116
A replay of the webcast will be available following the live events in the “Investor” section of the Valneva website at www.valneva.com.
Institutional investors who would like to meet with Valneva management at any of the below conferences are asked to submit a request to their representative at the respective bank.
About Valneva SE
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions.
We have a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently market three proprietary travel vaccines, including the world’s first and only chikungunya vaccine, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, the world’s most clinically advanced Shigella vaccine candidate, as well as vaccine candidates against the Zika virus and other global public health threats. More information is available at www.valneva.com.
|
Valneva Investor and Media Contacts |
Joshua Drumm, Ph.D.
|
|
|
Forward-Looking Statements
This press release contains certain forward-looking statements relating to the business of Valneva, including with respect to the progress, timing, results and completion of research, development and clinical trials for product candidates and regulatory approval of product candidates. In addition, even if the actual results or development of Valneva are consistent with the forward-looking statements contained in this press release, those results or developments of Valneva may not be sustained in the future. In some cases, you can identify forward-looking statements by words such as “could,” “should,” “may,” “expects,” “anticipates,” “believes,” “intends,” “estimates,” “aims,” “targets,” or similar words. These forward-looking statements are based largely on the current expectations of Valneva as of the date of this press release and are subject to a number of known and unknown risks and uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievement expressed or implied by these forward-looking statements. In particular, the expectations of Valneva could be affected by, among other things, uncertainties and delays involved in the development and manufacture of vaccines, unexpected clinical trial results, unexpected regulatory actions or delays, competition in general, currency fluctuations, the impact of the global and European credit crisis, and the ability to obtain or maintain patent or other proprietary intellectual property protection. Success in preclinical studies or earlier clinical trials may not be indicative of results in future clinical trials. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements made in this press release will in fact be realized. Valneva is providing this information as of the date of this press release and disclaims any intention or obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Attachment