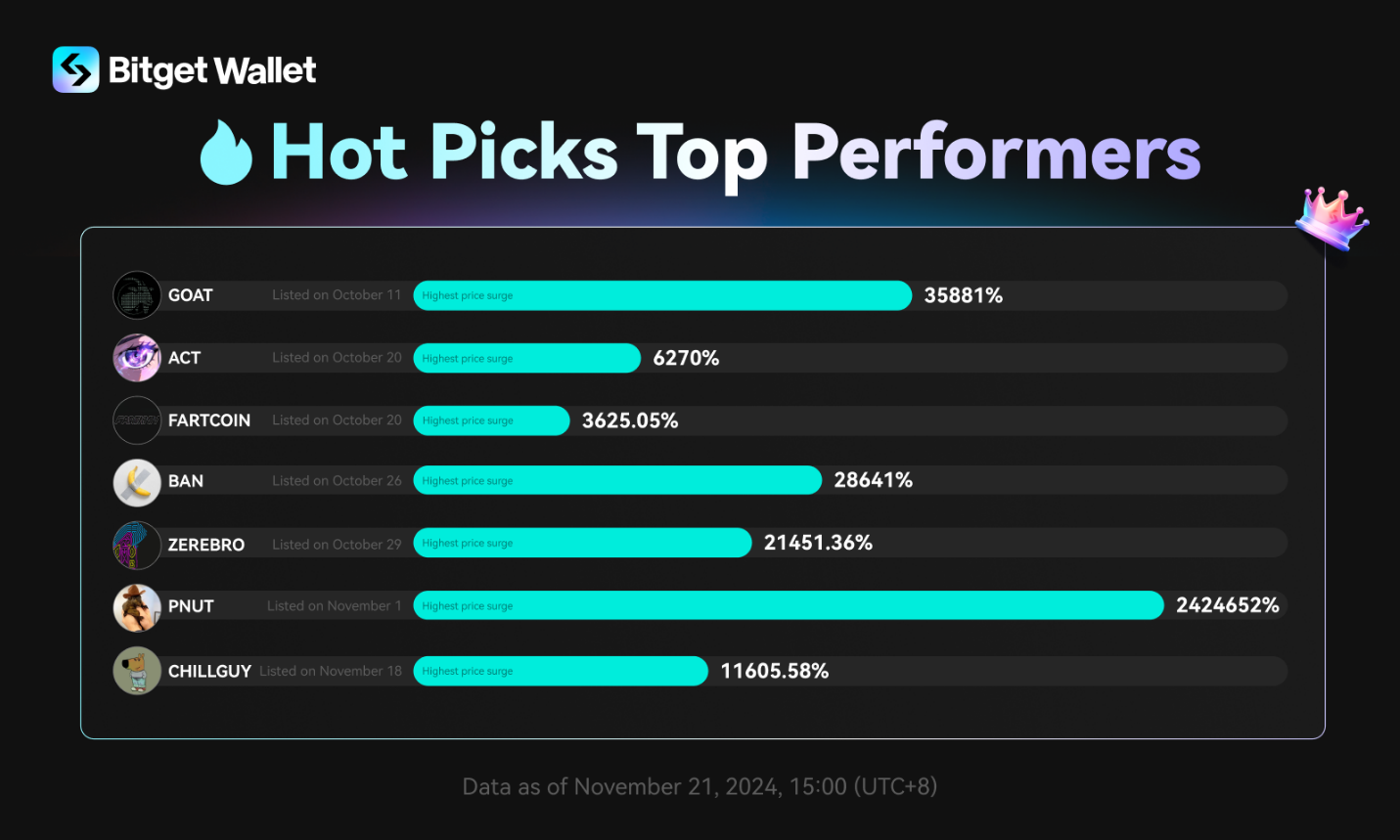
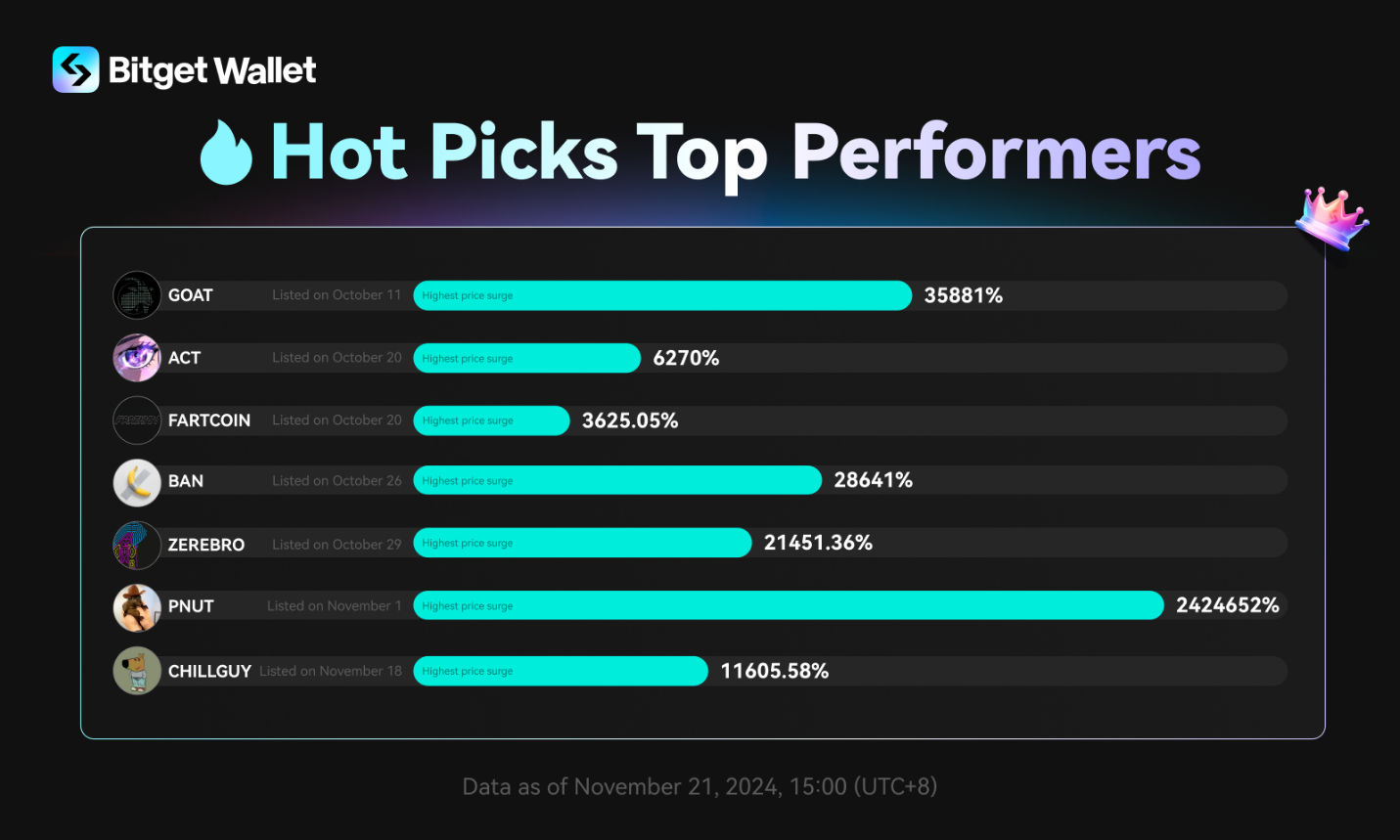
Jeito Capital co-leads €164.5 million ($181.4 million) oversubscribed Series D financing in Alentis Therapeutics to advance clinical development of Anti-Claudin-1 ADCs in solid tumors
Jeito Capital co-leads €164.5 million1 ($181.4 million) oversubscribed Series D financing in Alentis Therapeutics to advance clinical development of
Anti-Claudin-1 (CLDN1) ADCs in solid tumors
- Building on its co-lead investments in Alentis Therapeutics’ Series B (2021) and Series C (2023) rounds, Jeito’s renewed commitment demonstrates its strategy of supporting promising portfolio companies through key value-creation milestones
- Financing will advance next-generation targeted therapies for CLDN1 positive tumors
Paris, France, November 12, 2024 – Jeito Capital (“Jeito”), a global leading independent Private Equity fund dedicated to biopharma, announced today that it is co-leading a €164.5 million ($181.4 million) oversubscribed Series D financing round in Alentis Therapeutics (“Alentis”), a clinical-stage biotechnology company developing treatments for CLDN1 positive (CLDN1+) tumors and organ fibrosis. The financing will support Alentis in advancing its extensive pipeline of CLDN1+ targeted medicines for solid tumors.
The financing was led by new investor, OrbiMed, with existing investors Jeito Capital and Novo Holdings as co-leads. New international investors joining the round include Frazier Life Sciences, Longitude Capital, Catalio Capital, Piper Heartland Healthcare Capital and Avego Bioscience Capital. Existing investors RA Capital Management, Morningside Venture Investments, BB Pureos, Bpifrance through its InnoBio 2 fund and other early institutional investors also participated.
This investment marks Jeito’s third round of funding in Alentis, following its co-lead roles in the Series B (June 2021) and Series C (April 2023) financings. Since Jeito’s initial investment, Alentis has successfully progressed from early clinical trials to advancing multiple therapeutic programs. These successive investments reflect Jeito’s approach of providing both substantial capital and expert guidance to portfolio companies as they achieve key development milestones. This model has been successfully implemented across a significant part of Jeito’s portfolio, where continued investments are supporting the development of promising treatments in areas of high unmet need.
The proceeds will advance clinical development of Alentis’ two first-in-class Anti-CLDN1 ADC programs. The FDA recently cleared an IND application for the lead program ALE.P02, incorporating a tubulin inhibitor, with Phase 1/2 clinical trials in advanced or metastatic CLDN1+ squamous solid tumors set to begin in Q1 2025. Clinical development will also commence for the second program, ALE.P03 with a topoisomerase I inhibitor, with first-in-human trials planned for 2025. The financing will additionally support further pipeline development and general corporate purposes.
Dr. Rafaèle Tordjman, MD, PhD, Founder and CEO of Jeito Capital said: “We are excited to co-lead this major new financing round for Alentis, which further validates the strength of its pipeline and its exceptional innovation potential. This potential, combined with the company’s progress since our initial investment three years ago, reinforces Jeito’s refinancing strategy aimed at continuously supporting our promising portfolio companies to accelerate the development of breakthrough treatments with meaningful benefits for patients. We look forward to continuing to bring our expertise to support their development.”
Dr. Roberto Iacone, CEO of Alentis Therapeutics added: “This financing is a testament to the transformational potential of CLDN1 antibody-drug conjugates (ADCs) for the treatment of solid tumors. Let me take this opportunity to extend a warm welcome to our new investors. We’re excited to execute our development strategy and deliver clinical data for our programs over the next 12-18 months.”
About Jeito Capital
Jeito Capital is a global leading Private Equity fund with a patient benefit driven approach that finances and accelerates the development and growth of ground-breaking medical innovation. Jeito empowers and supports managers through its expert, integrated, multi-talented team and through the investment of significant capital to ensure the growth of companies, building market leaders in their respective therapeutic areas with accelerated patients’ access globally, especially in Europe and the United States. Jeito Capital has €534 million under management and a rapidly growing portfolio of investments. Jeito Capital is based in Paris with a presence in Europe and the United States.
For more information, please visit www.jeito.life or follow us on LinkedIn or X.
About Alentis Therapeutics
Alentis Therapeutics, the CLDN1 company, is a clinical-stage biotech developing breakthrough treatments for CLDN1+ tumors. CLDN1 is a previously unexploited target that plays a key role in the pathology of cancer and fibrotic disease. Alentis is the leading company pioneering anti-CLDN1 ADCs and antibodies to modify and reverse the course of select diseases.
Alentis was founded based on ground-breaking research in the laboratory of Prof. Thomas Baumert, MD at the University of Strasbourg and the French National Institute of Health and Medical Research (Inserm). Alentis is headquartered at the pharma-biotech hub in Basel, Switzerland with an R&D subsidiary in Strasbourg, France and clinical operations in the US.
Visit www.alentis.ch
Contacts:
Jeito Capital
Rafaèle Tordjman, Founder & CEO
Jessica Fadel, EA
Tel: +33 6 33 44 25 47
Maior
Stéphanie Elbaz – Tel: +33 6 46 05 08 07
ICR Healthcare
Mary-Jane Elliott / Davide Salvi / Kris Lam
Jeito@consilium-comms.com
Tel: +44 (0) 20 3709 5700
1 €164.5 million considering CHF 154.1 million raised and exchange rate of 1 EUR = CHF 0.9368 as of closing date (source: Banque de France)