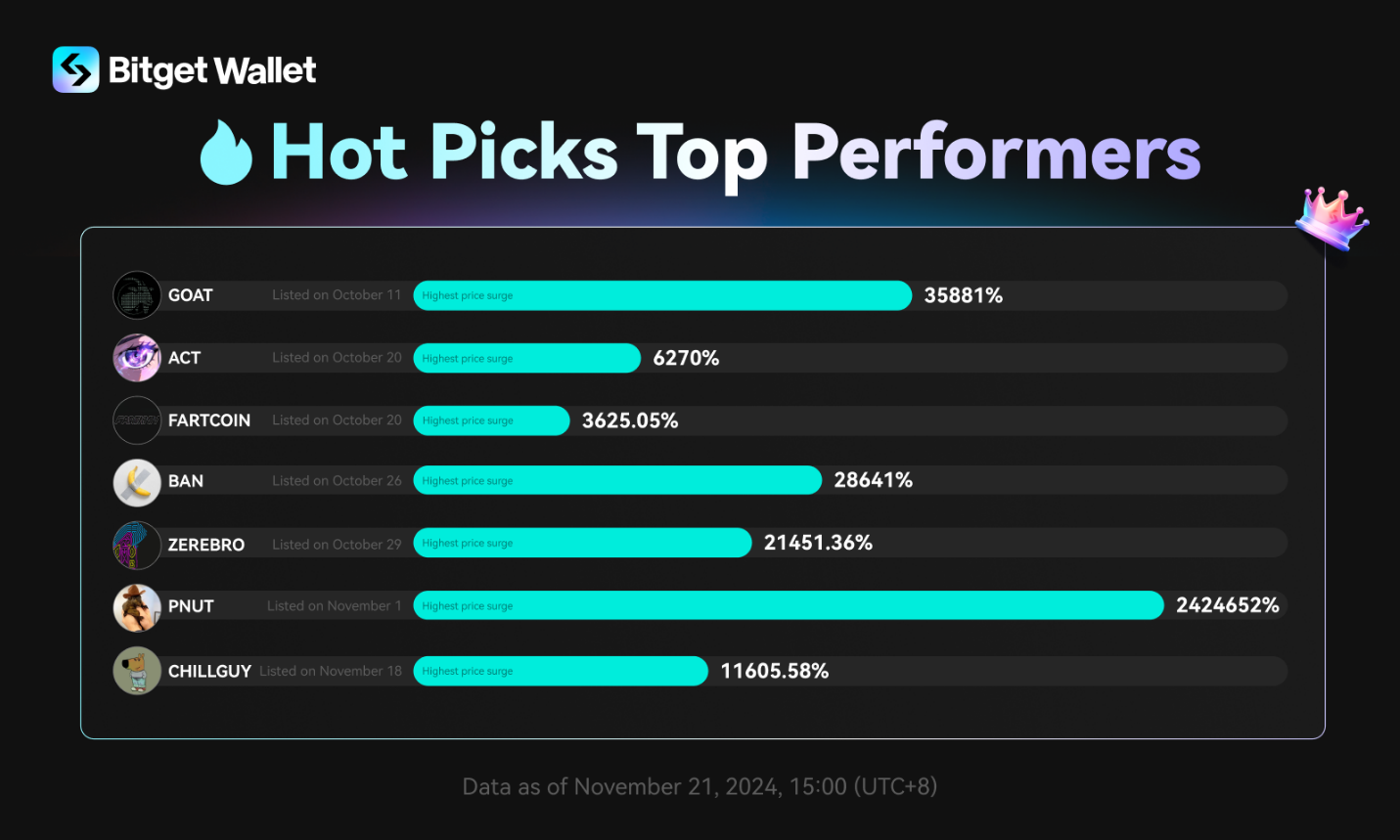
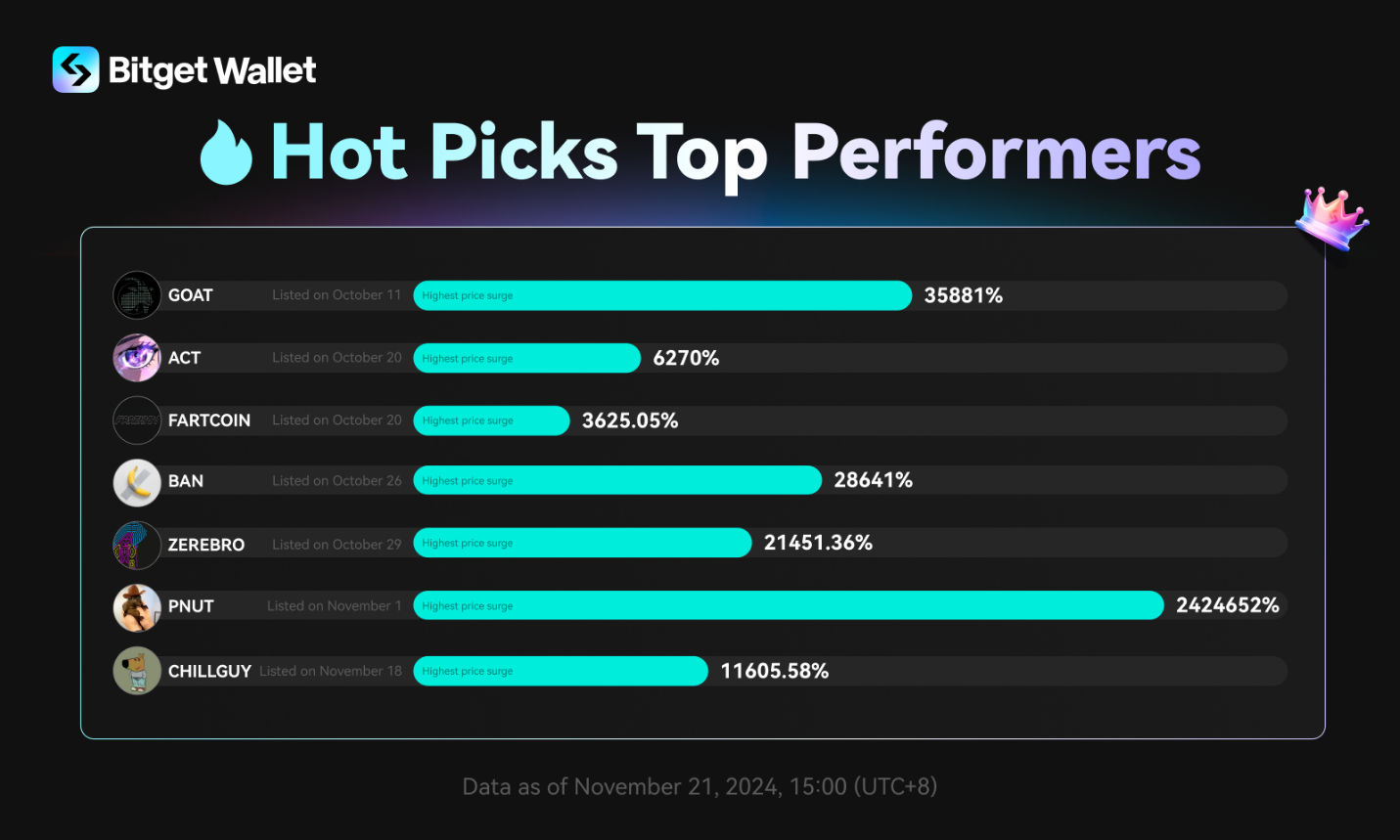
Dr. Joshua Weiser’s Proposed Legislation Addresses Federal Stem Cell Research Funding
Attorney Dr. Joshua Weiser has published a book proposing guidelines for embryonic stem cell research regulation in the United States. His work aims to influence federal laws to allow federal funding while balancing ethical considerations with scientific progress, potentially positioning the U.S. as a leader in regenerative medicine.

Photo Courtesy of Springer Publishing
NEW YORK, Nov. 12, 2024 (GLOBE NEWSWIRE) — Attorney Dr. Joshua Weiser has written a book that examines the future of embryonic stem cell research in the United States. His work aims to impact federal laws, address the ongoing debate over federal funding, advance research, and support medical progress.
Proposing a Path for Federal Funding in Medical Innovation
“We’re working to create guidelines that balance the ethical issues with the potential of embryonic stem cell research,” Joshua Weiser says. “The goal is to make federal funding possible while keeping research practices responsible and humane.” In his book, “Embryonic Stem Cells and the Law: Crafting A Humane System of Regulation,” Dr. Weiser outlines a detailed outlook on regulating research on stem cells.
Key recommendations include forming a national oversight committee, setting clear guidelines for informed consent from embryo donors, establishing strict protocols for using embryonic stem cell lines, and establishing ongoing ethical reviews and transparency in research practices.
The goal is to move research forward responsibly while addressing the ethical concerns surrounding this research.
Bridging Science and Ethics: Joshua Weiser’s Perspective on Stem Cell Laws
Joshua Weiser has developed a deep understanding of embryonic stem cell laws as part of a small group of attorneys specializing in this area at federal, state, and international levels. His research has gathered a comprehensive set of laws from around the world, which forms the basis of his recommendations.
“We’ve studied legal systems globally to create a solution that follows international practices while addressing the specific needs and concerns of the U.S.,” Dr. Weiser says.
The proposed guidelines balance scientific advancement and ethical concerns. They consider the moral questions surrounding embryos while acknowledging the potential of stem cell research to improve lives. These guidelines aim to gain support from the scientific community and those who have expressed ethical concerns about this research.
A Call to Action for Stakeholders
As discussions around funding for embryonic stem cell research continue, Joshua Weiser’s work offers a way forward. It seeks to address ethical concerns while supporting scientific progress, potentially influencing future legislation.
“Stem cells hold promise for the future of medicine,” Dr. Weiser said. “This research takes steps in that direction while considering ethical considerations. It’s an opportunity for the U.S. to advance responsibly and help those affected by untreatable diseases.”
About Joshua Weiser
Dr. Joshua Weiser is a renowned attorney who specializes in embryonic stem cell laws. He holds a JSD, the highest degree attainable by an attorney. He recently published the book Embryonic Stem Cells and the Law: Crafting A Humane System of Regulation with Springer. Dr. Weiser’s proficiency spans state, federal, and international stem cell legislation, making him a leading voice in this complex and evolving field.
Contact Information:
Contact Person’s Name: Joshua Weiser
Company: Weiser Health Law
Website: Weiserhealthlaw.com
Email: joshua@weiserhealthlaw.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f4a166b0-cf0a-4437-92e2-fc1342e1c22a