Tetanus Clinical Trial Pipeline Insights Featuring 20+ Companies | DelveInsight
Tetanus is a serious bacterial infection caused by Clostridium tetani, which affects the nervous system, leading to muscle stiffness and spasms. With an increased awareness of disease prevention, there is a rising demand for vaccines and boosters among general populations, especially among travelers and those exposed to occupational risks.
New York, USA, Nov. 06, 2024 (GLOBE NEWSWIRE) — Tetanus Clinical Trial Pipeline Insights Featuring 20+ Companies | DelveInsight
Tetanus is a serious bacterial infection caused by Clostridium tetani, which affects the nervous system, leading to muscle stiffness and spasms. With an increased awareness of disease prevention, there is a rising demand for vaccines and boosters among general populations, especially among travelers and those exposed to occupational risks.
DelveInsight’s ‘Tetanus Pipeline Insight 2024‘ report provides comprehensive global coverage of pipeline tetanus therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the tetanus pipeline domain.
Key Takeaways from the Tetanus Pipeline Report
- DelveInsight’s tetanus pipeline report depicts a robust space with 20+ active players working to develop 20+ pipeline tetanus drugs.
- Key tetanus companies such as G C Pharma, KM Biologics, Trinomab Biotech, Beijing Zhifei Lvzhu Biopharmaceutical Co., Ltd, Sinovac, Life Sciences Genrix Biopharmaceuticals, Changchun BCHT Biotechnology Co., and others are evaluating new tetanus drugs to improve the treatment landscape.
- Promising pipeline tetanus therapies such as GC 3111A, KD-370, TNM 002, Adsorbed diphtheria tetanus acellular pertussis combined vaccine, Tetanus vaccine, GR 2001, CBL 8851, and others are under different phases of tetanus clinical trials.
- In October 2024, the LENOWISCO and Cumberland Plateau Health Districts are offering free Tetanus, Diphtheria, Pertussis (Tdap) vaccines to people affected by Hurricane Helene’s flooding. Tetanus bacteria is naturally found in soil, and those wading through flood water could encounter sharp objects and sustain injuries allowing the bacteria to enter their body, the VDH said in a news release.
- In June 2024,. Recently, the findings of a non-peer-reviewed study reveal a significant reduction in PD occurrence following anti-tetanus vaccination, with a time-dependent association between the elapsed time since vaccination and the rate and progression of PD.A large-scale observational study in Leumit Health Services in Israel selected 1,446 patients who received a PD diagnosis between the ages of 45 and 75. This study found that 1.6% of those with PD had received the tetanus vaccine before their diagnosis, compared with 3.2% of those without. And no study participant developed PD within two years of being immunized. This study’s findings suggest that vaccination against tetanus neurotoxin (active or passive), possibly combined with treatments aimed at eradicating C. tetani from body reservoirs, could offer promising avenues to prevent PD occurrence and slow disease progression.
Request a sample and discover the recent advances in tetanus drugs @ Tetanus Pipeline Report
The tetanus pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage tetanus drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the tetanus clinical trial landscape.
Tetanus Overview
Tetanus is unique among vaccine-preventable diseases because it does not spread from person to person. The bacteria that cause tetanus are typically found in soil, dust, and manure, entering the body through skin breaks such as cuts or puncture wounds from contaminated objects. In the U.S., tetanus is rare, with only around 30 cases reported annually. Most cases occur in individuals who have not received the full series of recommended tetanus vaccinations, including those who have never been vaccinated and adults who are not up to date on their 10-year booster shots.
Tetanus is caused by the bacterium Clostridium tetani, whose spores are prevalent in the environment. When these spores enter the body, they turn into active bacteria. They typically invade through broken skin, especially from contaminated injuries like dirty wounds, punctures from nails or needles, burns, crush injuries, or injuries with dead tissue. The incubation period—the time between exposure and onset of symptoms—usually ranges from 3 to 21 days, with an average of 10 days, although it can vary from a day to several months depending on the wound type. In general, shorter incubation periods are associated with more contaminated wounds, more severe illness, and poorer outcomes.
Find out more about tetanus drugs @ Tetanus Analysis
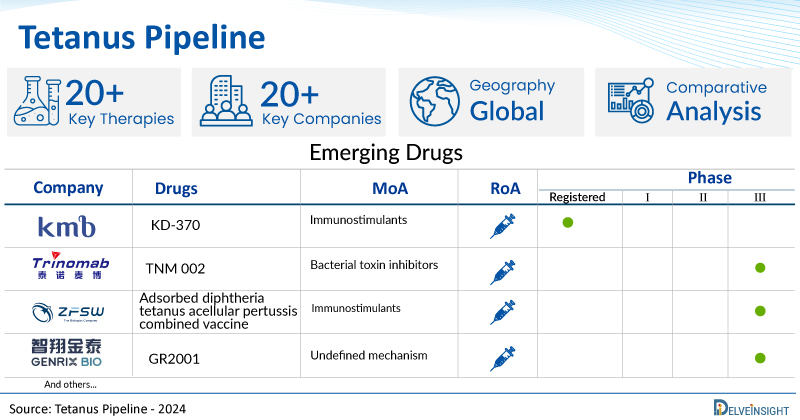
A snapshot of the Pipeline Tetanus Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| KD-370 | KM Biologics | Registered | Immunostimulants | Subcutaneous |
| TNM 002 | Trinomab Biotech | III | Bacterial toxin inhibitors | Intramuscular |
| Adsorbed diphtheria tetanus acellular pertussis combined vaccine | Beijing Zhifei Lvzhu Biopharmaceutical Co., Ltd | III | Immunostimulants | Intramuscular |
| GR2001 | Genrix (Shanghai) Biopharmaceuticals | III | Undefined mechanism | Intramuscular |
| GC 3111A | GC Pharma | II | Immunostimulants | Intramuscular |
Learn more about the emerging tetanus therapies @ Tetanus Clinical Trials
Tetanus Therapeutics Assessment
The tetanus pipeline report proffers an integral view of the emerging tetanus therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Tetanus Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: Immunostimulants, Bacterial toxin inhibitors
- Key Tetanus Companies: G C Pharma, KM Biologics, Trinomab Biotech, Beijing Zhifei Lvzhu Biopharmaceutical Co., Ltd, Sinovac, Life Sciences Genrix Biopharmaceuticals, Changchun BCHT Biotechnology Co. and others.
- Key Tetanus Pipeline Therapies: GC 3111A, KD-370, TNM 002, Adsorbed diphtheria tetanus acellular pertussis combined vaccine, Tetanus vaccine, GR 2001, CBL 8851 and others.
Dive deep into rich insights for new tetanus treatments, visit @ Tetanus Drugs
Table of Contents
| 1. | Tetanus Pipeline Report Introduction |
| 2. | Tetanus Pipeline Report Executive Summary |
| 3. | Tetanus Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Tetanus Clinical Trial Therapeutics |
| 6. | Tetanus Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Tetanus Pipeline: Late-Stage Products (Phase III) |
| 8. | Tetanus Pipeline: Mid-Stage Products (Phase II) |
| 9. | Tetanus Pipeline: Early-Stage Products (Phase I) |
| 10. | Tetanus Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Tetanus Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Tetanus Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the tetanus pipeline therapeutics, reach out @ Tetanus Therapeutics
Related Reports
Diphtheria Epidemiology Forecast
Diphtheria Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and diphtheria epidemiology trends.
Diphtheria Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diphtheria companies, including Sanofi-Pasteur, GlaxoSmithKline, Serum Institute of India, Bharat Biotech, Merck & Co., AJ Vaccines A/S, BIONET-ASIA, Meiji Holdings Co., Ltd., among others.
Diphtheria Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key diphtheria companies, including Sanofi-Pasteur, GlaxoSmithKline, Serum Institute of India, Bharat Biotech, Merck & Co., AJ Vaccines A/S, BIONET-ASIA, Meiji Holdings Co., Ltd., among others.
Pertussis Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key pertussis companies, including Serum Institute of India, LG Chem, ILiAD Biotechnologies, Dynavax Technologies, Tianjin CanSino Biotechnology, Faron Pharmaceuticals, Kymab, BioNet, among others.
Pertussis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key pertussis companies, including Tianjin CanSino Biotechnology, ILiAD Biotechnologies, LG Chem, BioNet-Asia, Intravacc, VAXFORM LLC, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com















